Two years ago this month, Kaiser Health News (KHN) released an investigative article critical of the U.S. Food and Drug Administration (FDA). It revealed severe and dangerous shortcomings in the agency’s reporting processes.
On March 7, 2019, the publication reported the FDA kept valuable information concerning injuries associated with medical devices hidden from view, and that included numerous reports of injuries sustained from surgical staplers.
The article reported that the FDA used a program based on alternative summary reports (ASRs). For years, the agency essentially allowed manufacturers to report information involving malfunctions and medical devices’ injuries to the ASR database. However, that database was kept from public view. In other words, when an individual, even if it was a doctor, wanted to search for information about the safety of a device, they did not have access.
The numbers were staggering. For example, Kaiser found that the ASR reporting, between 2016 and 2019, had 1.1 million incidents related to about 100 medical devices that the public could never access. Examples of medical devices ranged from blood glucose meters, breast implants and surgical staplers to dental implants and pacemakers.
And when it came to numbers involving surgical staples alone, Kaiser reported that just in the year 2016, “...nearly 10,000 malfunction reports were included in the hidden database.’’
Along with earning Kaiser Health News the prestigious Barlett & Steel Award for Investigative Journalism, the report spurred officials at the FDA to reorganize their system. The agency quickly shut the ASR database program down and created a new accessible database. At the same time, the agency also communicated with healthcare workers, ensuring they understood the high numbers of injuries and malfunctions. Between January 1, 2011, and March 31, 2018, there were 41,000 individual events involving staplers, including 366 deaths, over 9,000 serious injuries, and over 32,000 malfunctions.
The FDA also sent out a letter to the surgical stapler manufacturers, giving them instructions on labeling to further help the healthcare workers.
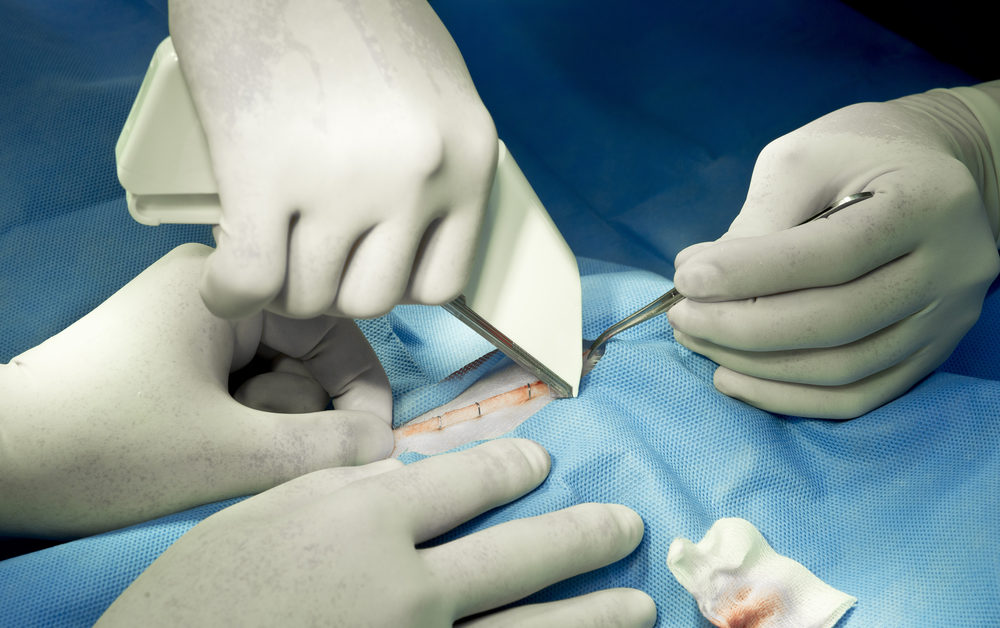
What makes surgical staplers dangerous?
When it comes to surgical staplers, there is little room for error. Injuries sustained by a surgical stapler usually occur in sensitive areas like the gastrointestinal tract, heart, lungs or kidneys. The damage associated with the injuries can include sepsis, internal bleeding, need for a permanent ostomy bag, and even death.
Here’s how they are used:
Surgical staplers are used during the removal of a part of an organ (resection), cutting through an organ or tissue (transection) or connecting structures together (anastomoses).
The process includes the surgeon loading a staple cartridge into the stapler, placing the tissue to be connected between the stapler jaws, and then activating the firing mechanism to shoot a staple into place. Some types of staplers also include a knife that transects tissue as the stapler is fired.
Along with human error, surgical stapler problems can involve weak material or dislodged parts that cause a misfiring. Deformed or defective staples, misapplied staples, or using the wrong size staples for a surgical procedure can also cause serious injuries.
Recalls and litigation in the last two years
The U.S. supply of surgical staplers comes mainly from two makers: Ethicon, a subsidiary of Johnson & Johnson, and Covidien, a subsidiary of Medtronic. Since the FDA changed its oversight process in 2019 and many malfunctions and errors have been revealed, both Ethicon and Medtronic have seen an increase in recalls of equipment, as well as lawsuits, including the following:
Medtronic: The manufacturer issued recalls for its surgical staplers, including the Endo GIA surgical stapler. And, in January of 2020, three different lawsuits were filed by plaintiffs against Medtronic. Each involved gastrointestinal surgeries that required staples to be precisely placed to reconnect the tissues after the surgery was completed. Complications included severe infections, cardiac problems, and the need for corrective surgeries.
One of the plaintiffs, an Iowa man, required several additional surgical treatments after a Medtronic Endo GIA stapler malfunctioned during the removal of a section of his bowel. One of the other plaintiffs, a Texas woman, endured the puncturing of her intestines when the Medtronic EEA surgical stapler allegedly misfired during her surgery. All three plaintiffs blame the manufacturers for these incidences. They state that the staplers were defectively designed and manufactured and that Medtronic failed to warn doctors and patients of the potential dangers.
Ethicon: In May 2019, the FDA announced a recall of Ethicon Endo-Surgery Intraluminal Staplers, identifying it as a Class 1 recall, which is the most serious type. In October 2019, Ethicon also recalled the Echelon Flex Powered Plus Endopath 60mm Stapler. Both were recalled due to the potential for malfunction.
To report a problem involving a surgical stapler, you can do so through the FDA’s MedWatch Adverse Event Reporting Program.










